How Many Electrons Can Exist in an Orbital
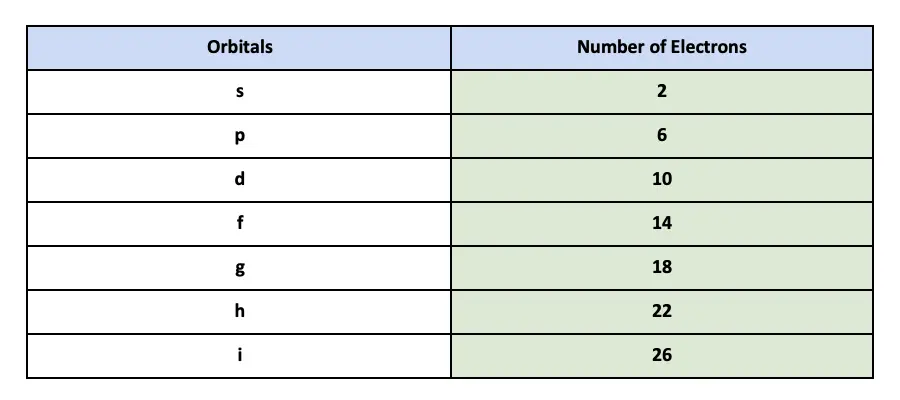
How Many Electrons Can An Orbital Hold. Therefore the p orbital can hold 6 electrons.
How Many Electrons Are In Each Shell Including 3p Orbitals
As a side note you can find the number of orbitals that can exist in a subshell by dividing the number of groups in a block by 2.

. 10 How many electrons maximum can have N 14 in the atom. Each orbital can contain a maximum of two electrons. This way that the s orbital can contain increase to 2 electrons the ns orbital can contain up to six electrons the d orbital deserve to contain as much as 10 electrons and the f orbital deserve to contain up to 14 electrons.
The 4p orbital holds 6 electrons. 31- Each orbital can hold a maximum of 2 electrons. N 5 ml 1.
By signing up youll get thousands of step-by-step solutions to your homework questions. In the second shell both 2s and 2p orbitals exist as it can have a maximum of 8 electrons. Cn 5 ms 12.
The five d orbitals can hold up to 10 electrons. N 3 can be up to 5. Of groups in the block 2.
Each orbital can hold up to two electrons meaning that the 1s 2s 3s 4s and 5s can hold two electrons. Since every orbital can hold a maximum of 2 electrons two electrons would be in the sigma orbital and the other. A 1 B 2 C 3 D 4 Answer.
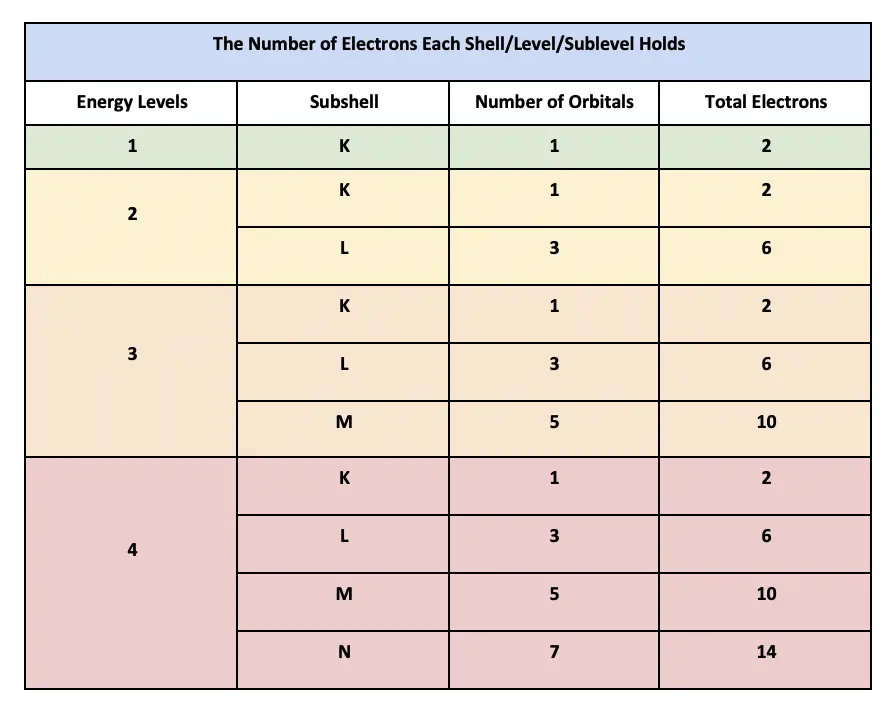
Thus the third level holds a maximum of 18 electrons. 11 How many orbitals are there in a subshell for which N 4 and L 3. Of orbitals in a subshell no.
2 in the s orbital 6 in the three p orbitals and 10 in the five d orbitals. The s subshell possesses one. 12 How many possible orbitals are there for N 3.
None of these Select one. A molecular orbital can hold two electrons so both electrons in the H 2 molecule are in the σ 1s bonding orbital. May 26 2014.
The subshells which include s p d and f the contain the orbitals. Also asked how many electrons can be placed in an antibonding molecular orbital. 14 How many electrons with N 5 can an atom contain.
Also how many electrons can the 4th orbital hold. How many electrons can an oral LD have. How many electrons can exist in an orbital.
Determine the number of electrons in an atom that can have the following quantum numbers. There is a difference between orbital and subshell. Helium has two 1s electrons therefore if two He atoms form a bond 4 electrons has to be placed into the molecular orbitals.
In the first shell there is only the 1s orbital as this shell can have a maximum of only 2 electrons. If n 1 then there is only 1 orbit. How many electrons can exist in an orbital.
This means that the s orbital can contain up to two electrons the p orbital can contain up to six electrons the d orbital can contain up to 10 electrons and the f orbital can contain up to 14 electrons. Therefore the 1p orbital doesnt exist. However the electron deserve to exist in spin increase ms 12 or with spin under ms -12 configurations.
The 2p3p 4p and 5p can each hold six electrons because they have three orbitals. When molecular orbitals form two valence electrons are required to be situated in between two atoms for a molecular orbital forming a chemical bond. 15 What is the maximum number of electrons in an atom that can have n 4 L 1 ml 0.
Degenerate orbitals exist the second electron shell where FAQhow many distinct and degenerate orbitals exist the second electron shell where adminSend emailDecember 16 2021 minutes read You are watching. How many electrons can exist in an orbital. The subshell that has three orbitals and can hold up to six electrons is the.
The fourth energy level of the periodic table includes the 4s 3d and 4p orbitals. An orbital can hold a pair of electrons each possessing a different spin. To calculate electron shell capability you first need to determine the number of electrons possible per shell then apply the 2n 2 formula.
How do you find the number of electrons in a molecular orbital. How many electrons will there be with the quantum number L 1Since 2p have 6 electrons in it and 3p have 2 electrons in it there are total of 8 electrons that have l1 as one of their quantum numbersWhat is the maximum number of electrons in an atom that can have the following quantum numbers n 3. The lowest energy orbital in the quantum-mechanical model is the.
The fourth energy level has 18 electrons. The third shell in its lowest state has room for 8 electrons but including the higher energy 3d electrons it has a capacity of 18 electrons. Which orbital would the electron of a ground state hydrogen atom occupy.
The electron configuration is σ1s2 σ 1 s 2. This is the case because an orbital can hold a maximum of 2 electrons as stated by the Pauli Exclusion Principle. 13 What orbitals are in the N 4 shell.
How many electrons can exist in an atomic orbital Note. How many electrons are unpaired in the orbitals of carbon. Orbitals exist the second electron shell where FAQhow many distinct orbitals exist the second electron shell where adminSend emailNovember 29.
N 2 can have up to 3 verbs. For example s - subshell has 1 orbital so it can hold 2 electrons p subshell View the full answer. And n 4 can have up to 7 verbs.
The p orbital has three sub levels with the possibility of two electrons in each suborbital. Click to see full answer. Electrons in this orbital are called s electrons and have the lowest energy of any electrons in that principal energy level.
A s subshell B p subshell C d subshell D f subshell. Students should confuse orbitals with the subshells which are different.

How Many Electrons Can The Third Energy Level Hold At Level
How Many Electrons Are In Each Shell Including 3p Orbitals
Electron Arrangement In Atoms Elements And The Periodic Table
0 Response to "How Many Electrons Can Exist in an Orbital"
Post a Comment